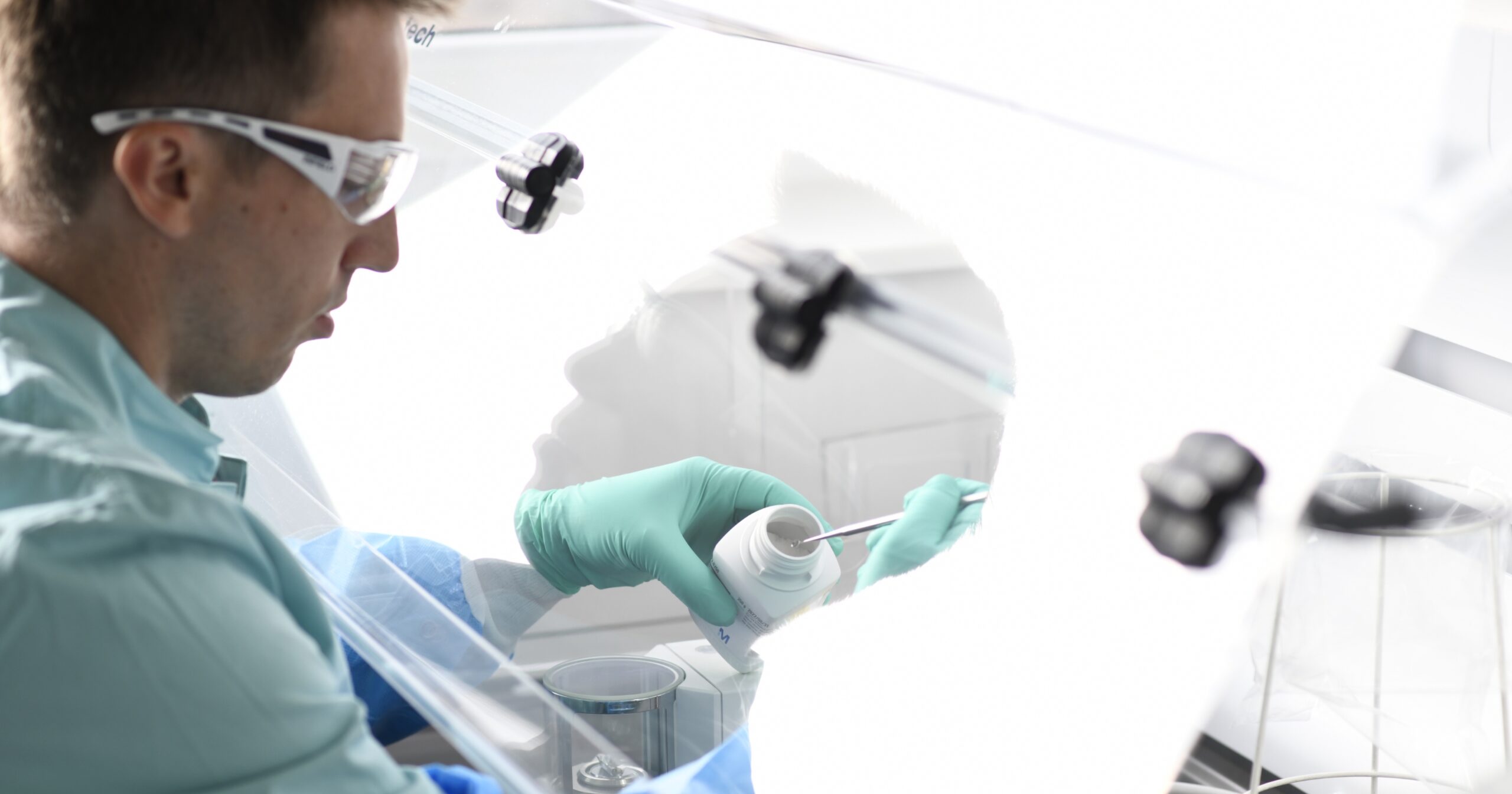
Tanja Schötz, chemical laboratory assistant
Our employees are highly qualified.
Excella has trained many chemical laboratory technicians. Some have come from other companies or laboratory sectors, a combination that we find extremely fruitful.
Tanja Schötz, chemical laboratory assistant